The software meets all requirements of FDA 21 CFR Part 11, GAMP5, Annex 11
regulations and presents some peculiar features meeting some requirements of
particular applications. It is dedicated to process and instrument,
particularly autoclaves, validation.
Features:
Specifications:
| Operating systems: | Windows 10, 11 (32, 64 bit) |
| Regulation compatibility: | FDA 21 CFR Part 11 |
| Data loggers supported: | SterilDisk, P-Micro, S-Micro, S-Disk J, MicroW, L-Disk, RHTemp, PressureDisk, S-Radio, Pirani acuum Logger, TecnoStick, HumiStick |
| Data management: | Client/server database with single missions, groups, customers, instruments, devices, programming profiles |
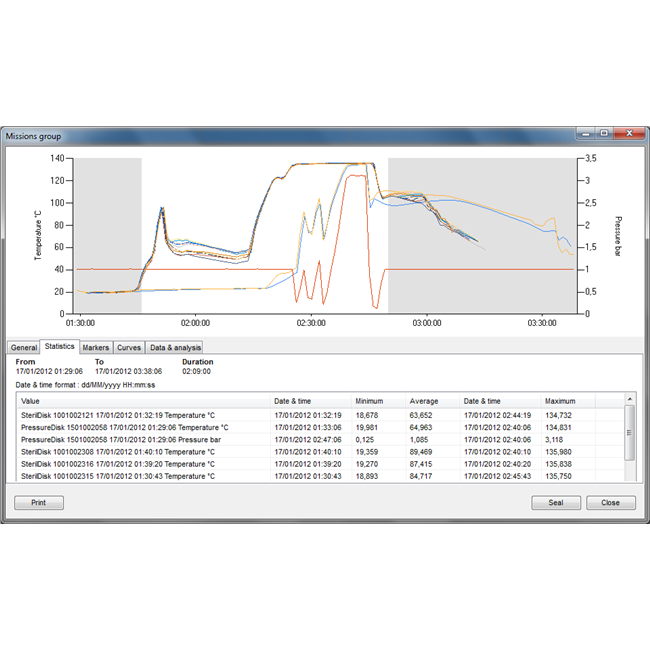
| Data display: | Graph (with zoom) and table (exportable into Excel and HTML), markers for process start and end, printed report of all the data, Multi graph |
| Customizable parameters: | Acquisition step, mission duration, analysis type (lethality with Z and reference temperature values setting, Overkilling for validation) |
| Calculated parameters: | Lethality (F0, PU. A0 etc.) |
| Accessories: | DiskInterface HS (Part #: TS0200), Universal Multibay (Part #: TS04MBU) , TecnoStick Interface (Part #: TS04IUSB) |
| Languages: | Italian, English, German |