|
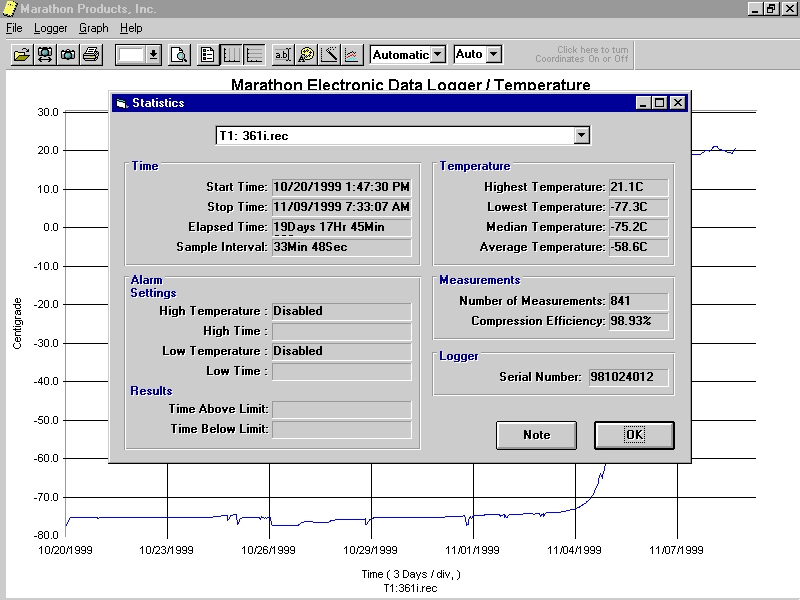
This new Marathon 21 CFR-Viewer READ ONLY Software works with all electronic Marathon dataloggers and meets FDA requirements for electronic records and electronic signatures used in the pharmaceutical, biotech and medical industry. It is designed to comply with the Code of Federal Regulations Section 21 CFR Part 11 as required by the FDA. Marathon 21 CFR DB validates data and works seamlessly with our Marathon Dataloggers. The software provides sophisticated data encryption and storage, administrative and multi-level user security profiles, as well as tools for manipulating and retrieving data. When used in conjunction with the Marathon dataloggers, the software provides a complete analytical solution for quality assurance standards.
|
|||||
|
|||||
| Specifications: | |||||
|
|||||
For use with ALL Marathon electronic dataloggers.